Organization & Main Tasks
Biopharmaceuticals and Herbal Medicines Bureau
Biopharmaceuticals and
Herbal Medicines Bureau
- Biopharmaceutical
Policy Division - herbal Medicines
Policy Division - Cosmetic
Policy Division - Quasi-drugs
Policy Division
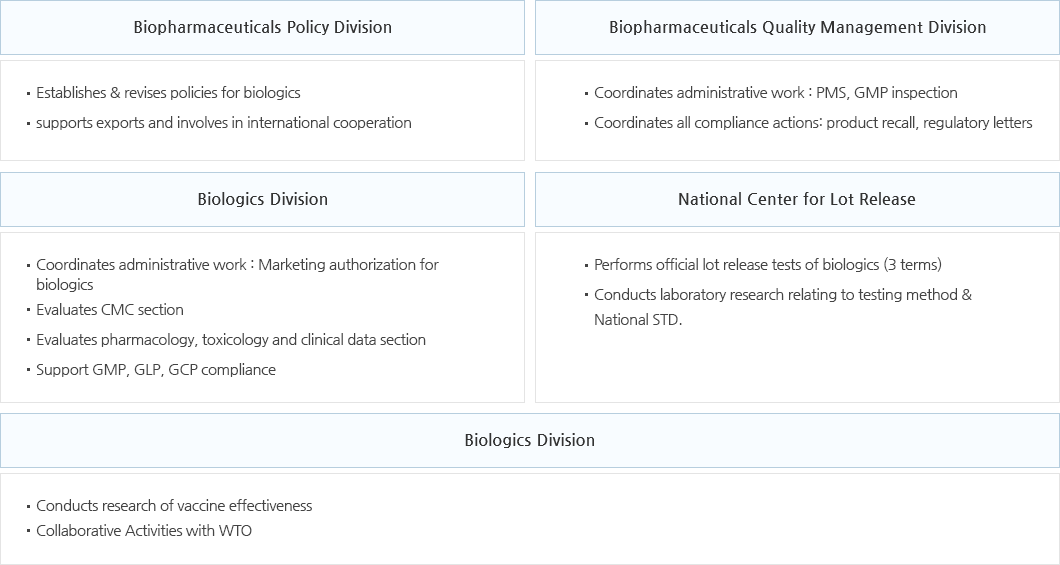
Biopharmaceutical Policy Division
- Establishes & coordinates safety management policies for biopharmaceuticals; creates & revises policies for biopharmaceuticals, transplantation materials and blood plasma
- Supports exports and involves in international cooperation
Biopharmaceutical Quality Management Division
- Establishes & runs comprehensive domestic/international inspection plans regarding biopharmaceutical manufacturing, quality management criteria and human transplantation materials management
- Establishes & coordinates biopharmaceutical supervision/monitoring plans; establishes/runs GMP training/capability building plans
Herbal Medicines Policy Division
- Establishes and coordinate policies; implement and revise rules and regulations; engage in international cooperation; grant market authorization of domestically-manufactured/imported products & develop relevant policies in herbal medicines
Cosmetics Policy Division
- Establishes and coordinate policies; implement & revise rules, regulations and notifications; make and coordinate monitoring plans in cosmetics products
Quasi-drugs Policy Division
- Establishes and coordinate policies; implement & revise rules, regulations and notifications; make and coordinate monitoring plans; grant market authorization of domestically-manufactured/imported products & develop relevant policies in quasi drugs
Biopharmaceuticals and Herbal Medicines Evaluation Department
Biopharmaceuticals and Herbal
Medicines Evaluation Department
- Biologics Division
- Recombinant
Product Division - Gene and Cell
Therapy Products Division - Herbal Medicines
Products Division - Cosmetics
Evaluation
Division
Biologics Division
- Reviews & evaluates quality, safety and efficacy of biologics & biopharmaceutical diagnostics products
- Approves domestic and import related products
Recombinant Products Division
- Reviews & evaluates quality, safety and efficacy of recombinants
- Approves domestic and import related products
Cell and Gene Therapy Products Division
- Reviews & evaluates quality, safety and efficacy of cell therapies, gene therapies, tissue-engineered products
- Approves domestic and import related products
Herbal Medicines Products Division
- Reviews & evaluates quality, safety and efficacy of herbal medicines
- Approves domestic and import related products
Cosmetics Evaluation Division
- Reviews & evaluates quality, safety and efficacy of cosmetics and quasi medicines
- Approves domestic and import related products
Control of Biologics
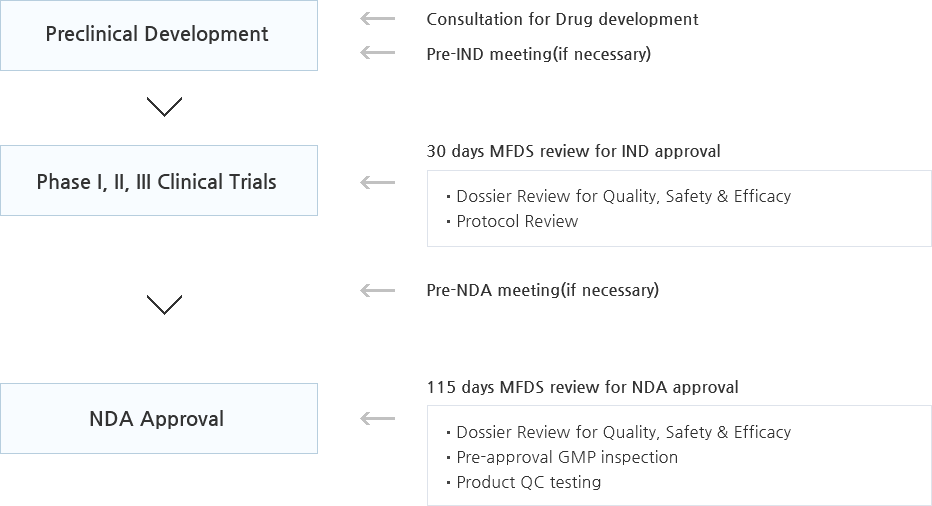
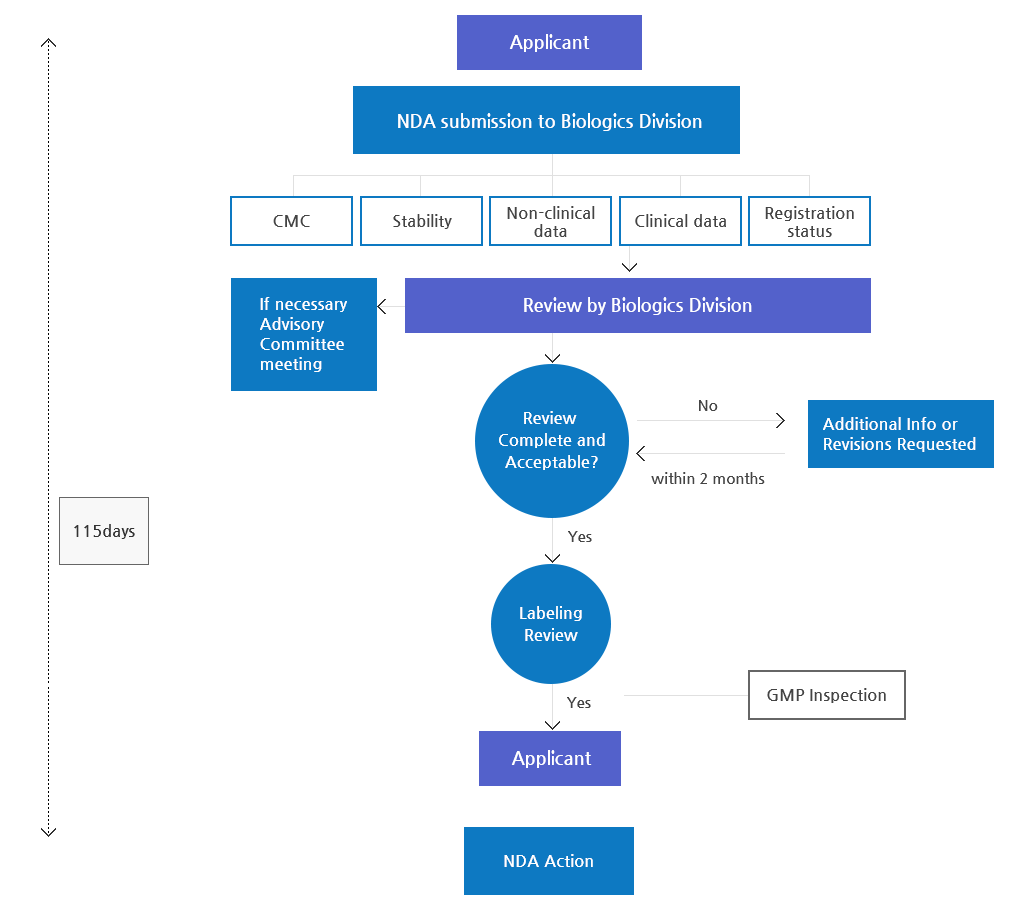
Approval Process
From Development to Authorization
Sponsors have submitted application as electronic documents through KiFDA online system since Oct. 2nd , 2006.
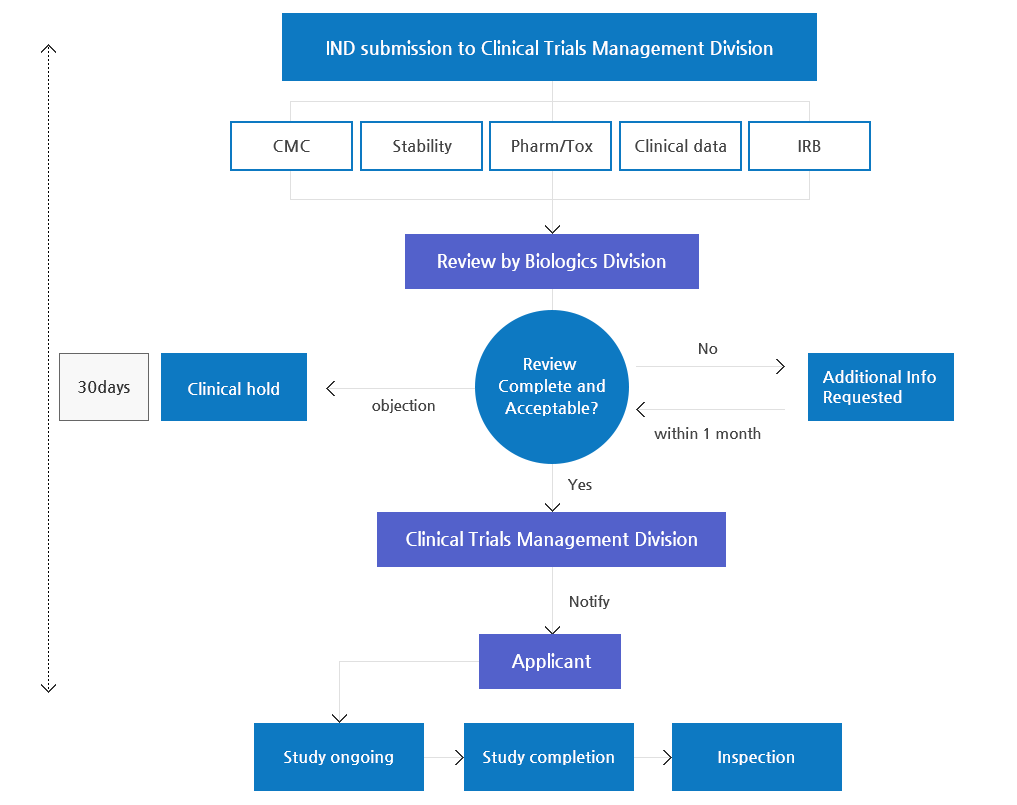
IND(Investigational New Drug Application) Review Process
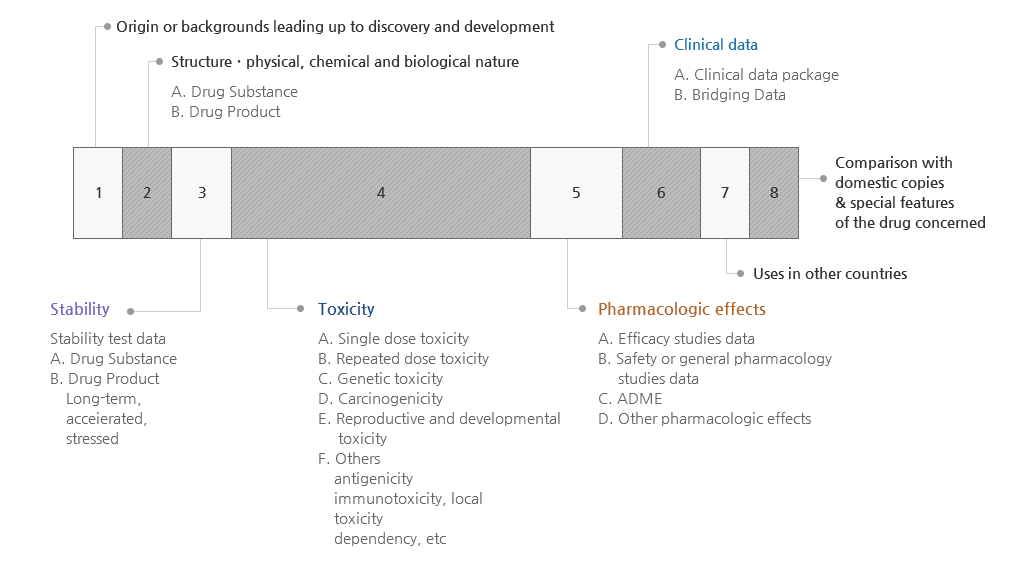
- Dossier for IND
- Development plan
- Introduction
- Data on structural identification and psychochemical and biological properties (including data for a placebo)
- Data on non-clinical studies
- Data on Pharmacology
- Data on Toxicity
- Data on clinical studies (if applicable)
- Study protocol
- References
- Investigator’s Brochure (IB)