EUA(Emergency Use Authorization) Process for COVID-19 in Korea
- Registration Date 2020-07-02
- Hit 6944
EUA(Emergency Use Authorization) Process for COVID-19 in Korea
1) EUA in Korea
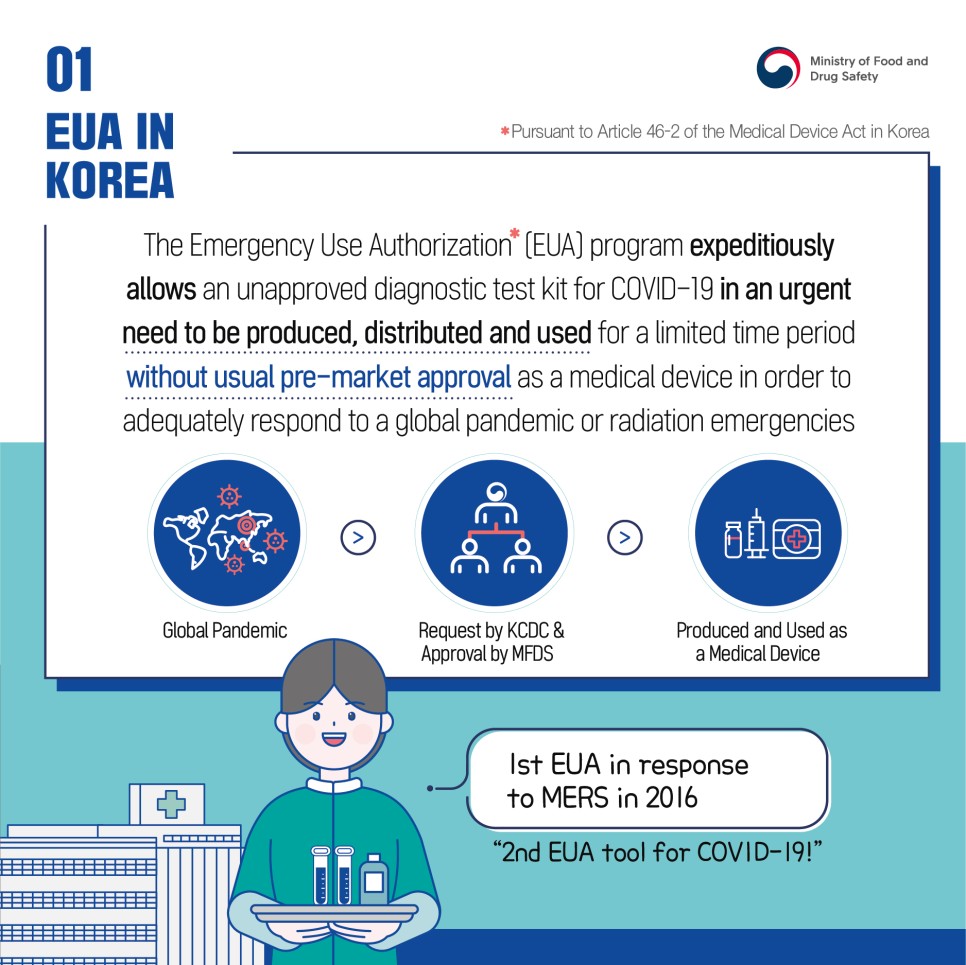
The Emergency Use Authorization* (EUA) program expeditiously allows an unapproved diagnostic test kit for COVID-19 in an urgent need to be produced, distributed and used for a limited time period without usual pre-market approval as a medical device in order to adequately respond to a global pandemic or radiation emergencies
*Pursuant to Article 46-2 of the Medical Device Act in Korea
Global Pandemic -> Request by KCDC & Approval by MFDS -> Produced and Used as a Medical Device
1st EUA in response to MERS in 2016
"2nd EUA tool for COVID-19!"
2) EUA process
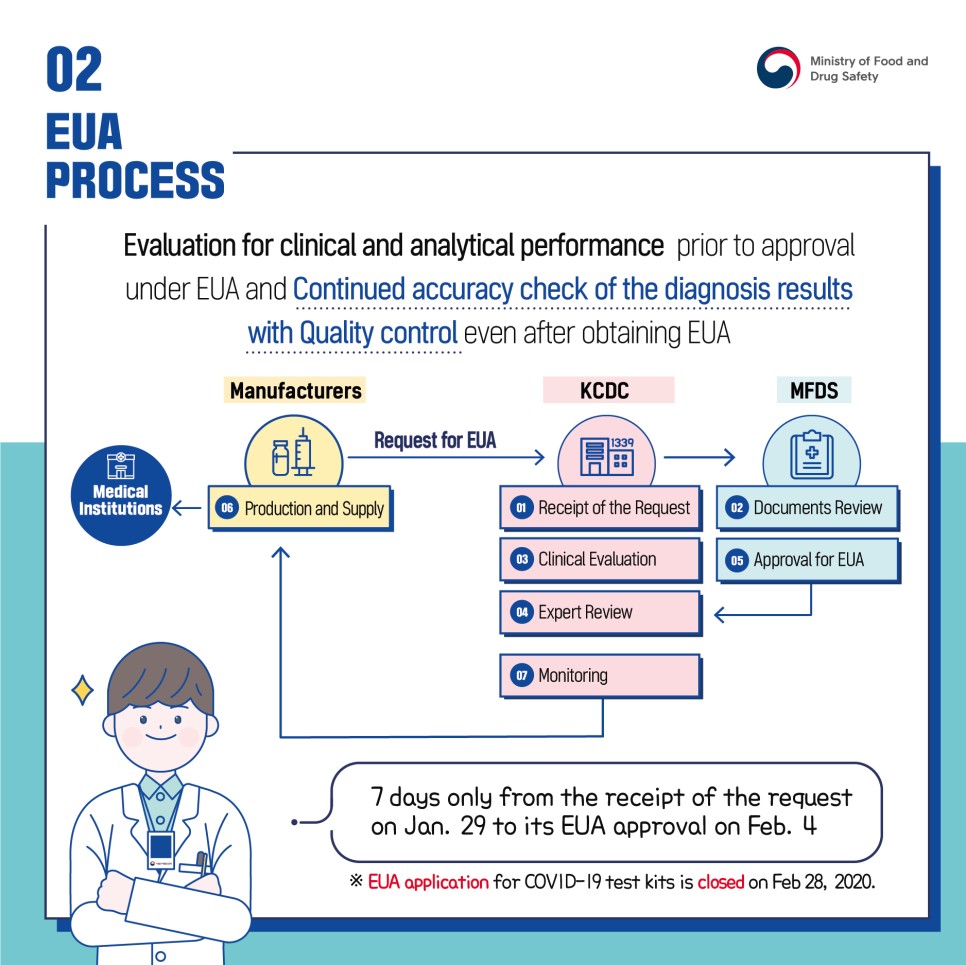
Evaluation for clinical and analytical performance prior to approval under EUA and Continued accuracy check of the diagnosis results with Quality control even after obtaining EUA
[Manufacturers] -> Request for EUA -> KCDC
6) Production and Supply -> Medical Institutions
[KCDC]
1) Receipt of the Request
3) Clinical Evaluation
4) Expert Review
7) Monitoring
[MFDS]
2) Documents Review
5) Approval for EUA
7 days only from the receipt of the request on Jan. 29 to its EUA approval on Feb. 4
*EUA application for COVID-19 test kits is closed on Feb 28, 2020.
3) Supply of the kits
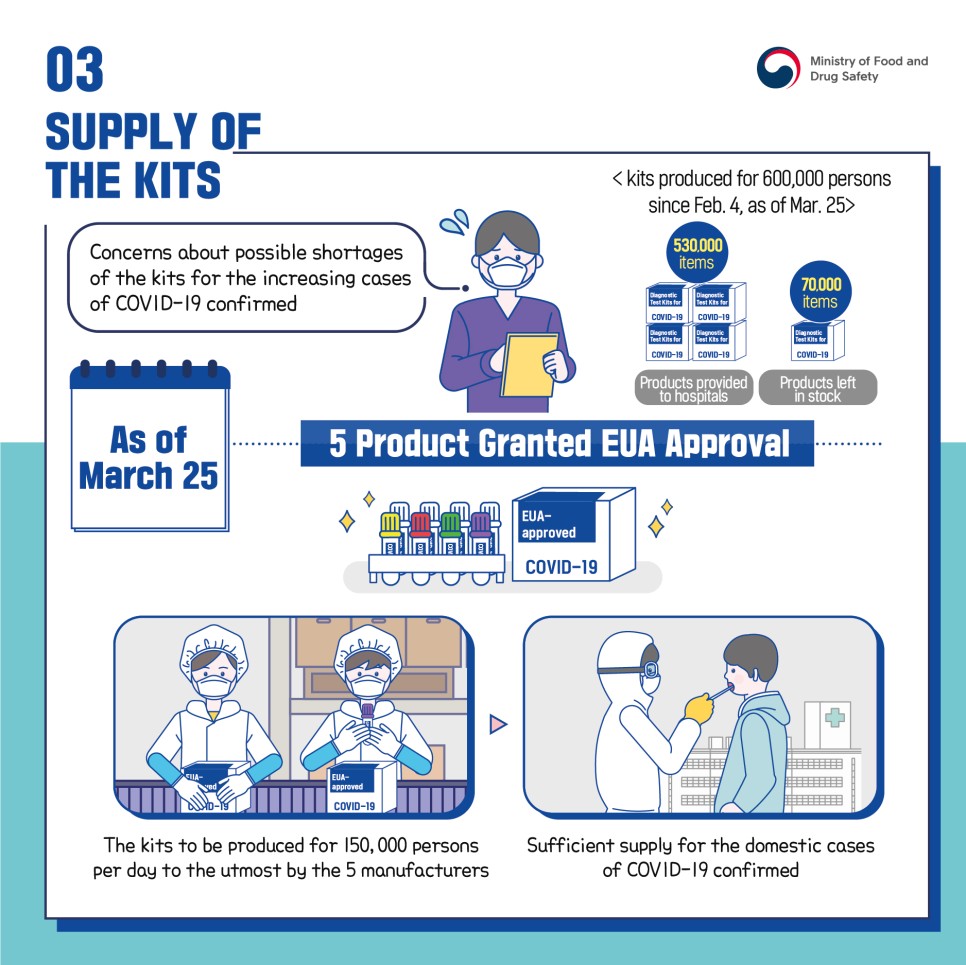
Concerns about possible shortages of the kits for the increasing cases of COVID-19 confirmed
As of March 25, 5 Products Granted EUA Approval
530,000 items (products provided to hospitals)
70,000 items (products left in stock)
The kits to be produced for 150,000 persons per day to the utmost by the 5 manufacturers
Sufficient supply for the domestic cases of COVID-19 confirmed
4) Government-supported exports
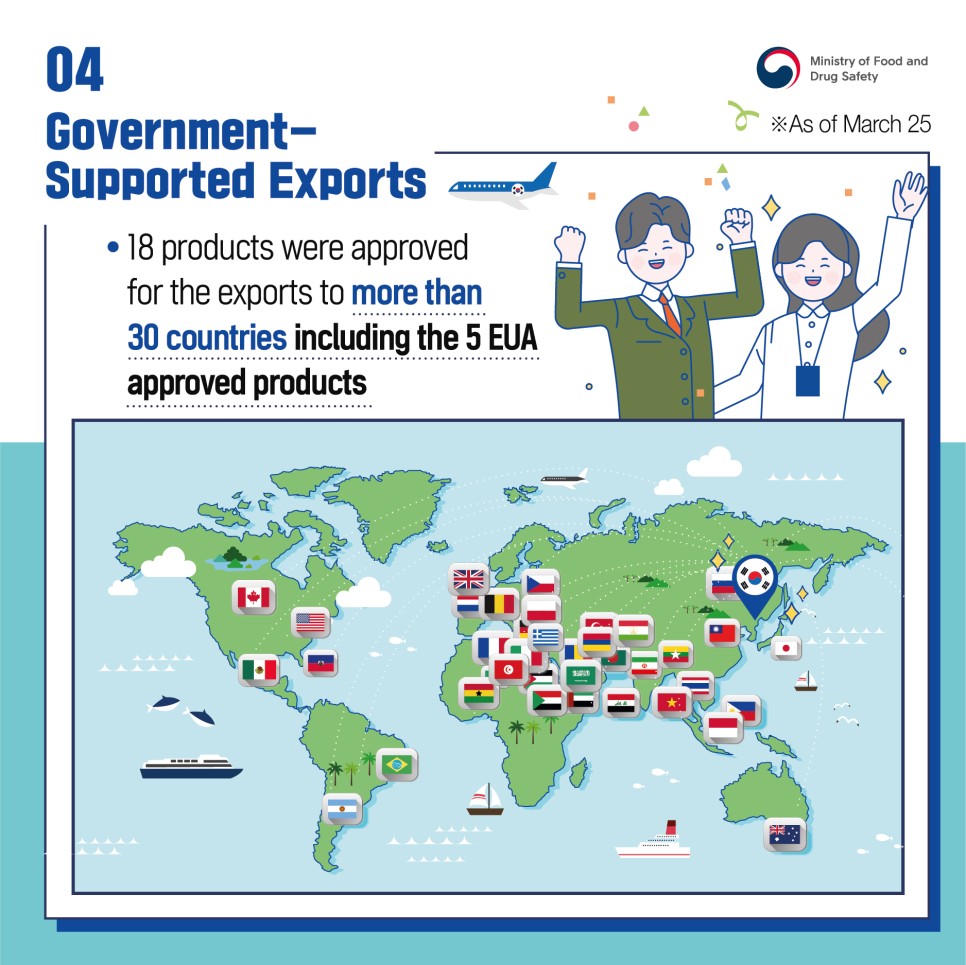
- 18 products were approved for the exports to more than 30 countries including the 5 EUA approved products
*As of March 25
Towards Transparency and Effectiveness
to Combat COVID-19 with K-Bio and MFDS
Please refer to the Press Release on March 26, 2020 for more information.
1) EUA in Korea
The Emergency Use Authorization* (EUA) program expeditiously allows an unapproved diagnostic test kit for COVID-19 in an urgent need to be produced, distributed and used for a limited time period without usual pre-market approval as a medical device in order to adequately respond to a global pandemic or radiation emergencies
*Pursuant to Article 46-2 of the Medical Device Act in Korea
Global Pandemic -> Request by KCDC & Approval by MFDS -> Produced and Used as a Medical Device
1st EUA in response to MERS in 2016
"2nd EUA tool for COVID-19!"
2) EUA process
Evaluation for clinical and analytical performance prior to approval under EUA and Continued accuracy check of the diagnosis results with Quality control even after obtaining EUA
[Manufacturers] -> Request for EUA -> KCDC
6) Production and Supply -> Medical Institutions
[KCDC]
1) Receipt of the Request
3) Clinical Evaluation
4) Expert Review
7) Monitoring
[MFDS]
2) Documents Review
5) Approval for EUA
7 days only from the receipt of the request on Jan. 29 to its EUA approval on Feb. 4
*EUA application for COVID-19 test kits is closed on Feb 28, 2020.
3) Supply of the kits
Concerns about possible shortages of the kits for the increasing cases of COVID-19 confirmed
As of March 25, 5 Products Granted EUA Approval
530,000 items (products provided to hospitals)
70,000 items (products left in stock)
The kits to be produced for 150,000 persons per day to the utmost by the 5 manufacturers
Sufficient supply for the domestic cases of COVID-19 confirmed
4) Government-supported exports
- 18 products were approved for the exports to more than 30 countries including the 5 EUA approved products
*As of March 25
Towards Transparency and Effectiveness
to Combat COVID-19 with K-Bio and MFDS
Please refer to the Press Release on March 26, 2020 for more information.
Division Director for International Cooperation
Written by Bae Seong-myeong
Telephone 043-719-1559