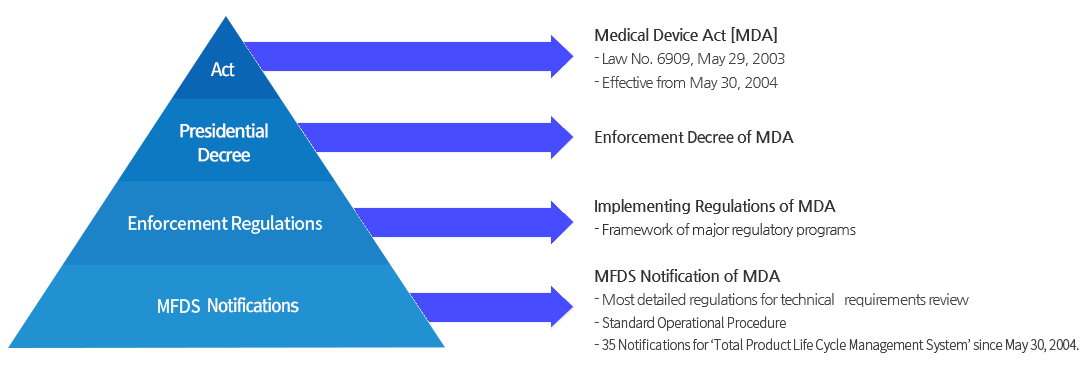
Regulations at Four hierarchical Orders
Regulations at Four hierarchical Orders
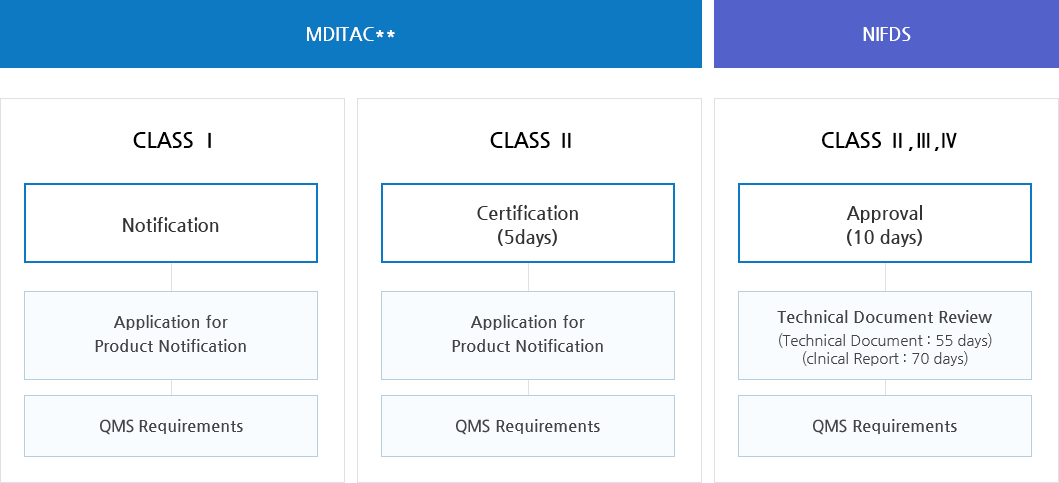
Overview of Notification, Certification and Approval Process in Medical Devices
MFDS requires the submission of 'Technical Documents' for the certification and approval of medical devices.
In principle, Class I & II devices are certified by ‘Medical Device Information and Technology Assistance Center(MDITAC) the ‘National Institute of Medical Device Safety Information (NIDS) and Class III & IV devices are approved by MFDS. However, Class I & II devices in categories below must be approved by MFDS.
> which require clinical test reports
> Digital Healthcare related (ex. telemedicine system)
> Undefined Nomenclature & classification regulation
> Combined with pharmaceuticals, etc.
In principle, Class I & II devices are certified by ‘Medical Device Information and Technology Assistance Center(MDITAC) the ‘National Institute of Medical Device Safety Information (NIDS) and Class III & IV devices are approved by MFDS. However, Class I & II devices in categories below must be approved by MFDS.
> which require clinical test reports
> Digital Healthcare related (ex. telemedicine system)
> Undefined Nomenclature & classification regulation
> Combined with pharmaceuticals, etc.
1. Technical Documents
- Documents related to quality of medical devices, such as performance and safety, etc.
- Which include information on ‘Intended Use’, ‘Mechanism of action(MoA)’, ‘Operational(Functional) Structure’, ‘Raw Materials’, ‘Instruction for Use’, ‘Test Specifications’, etc.
2. Technical document classification
- The technical documents is consist of ‘Application Form’ and ‘Supplementary Evidence’. The presence of mandatory submission of ‘clinical trial reports’(as a part of ‘Supplementary Evidence’) determines application procedure to be followed.
- 2.1 General Technical Document Review
- If a device is substantially equivalent legally marketed devices, ‘clinical trial reports’ are not required.
- 2.2 Safety and Efficacy Review(SER)
- The technical document including ‘clinical tiral reports’ is thoroughly reviewed
※ ‘Clinical trial reports’ are required if differences such as ‘Intended Use’, ‘Mechanism of Action(MoA)’ and ‘Raw Materials’ could significantly affect safety and efficacy of devices
- The technical document including ‘clinical tiral reports’ is thoroughly reviewed
3. Premarket Approval
- Class I (Notification)
- Class II (Certification, Approval)
- Class III, IV (Approval)
Classification of medical devices
- 4 classes(Ⅰ∼Ⅳ) based on potential risk to human health
- Harmonized with GHTF/IMDRF rules
- 2,109 Medical Device items, excluding IVDD(In-Vitro Diagnostic Devices) and 225 IVDD items are recognized pursuant to ‘Regulations for Product Classification of Medical Device and Class by Product’ and by ‘Regulations for Product Classification of In-Vitro Diagnostic Device and Class by Product’
【Medical Device (except IVD)】
| CLASS | RISK LEVEL | EXAMPLES |
NUMBER OF CLASSIFIED DEVICES |
|---|---|---|---|
| I | VERY LOW | Ophthalmic microscope, Radiation shielding glove, Operation table, Stethoscope, etc. | 521 |
| II | LOW | MRI, Pulse oximeter, Sterilizer, Electroencephalograph , etc. | 1,017 |
| III | MODERATE | Cryosurgical(mechanical) system, Anaesthesia(gas) system, Silk suture, Condom, etc. | 318 |
| IV | HIGH | Implantable defibrillator, Coronary stent, Biodegradable spine disc, Intraocular lens , etc. | 253 |
| TOTAL | 2,109 | ||
【IVD】
| CLASS | RISK LEVEL | EXAMPLES |
NUMBER OF CLASSIFIED DEVICES |
|---|---|---|---|
| I | VERY LOW | IVD reagents for extracting nucleic acids, Specimen transport media, Blood type(automation) analyzer, etc. | 93 |
| II | LOW | IVD reagents for urine chemistry, IVD reagents for vitamin test, IVD reagents for allergy test, etc. | 74 |
| III | MODERATE | IVD strip for glucose self test, IVD reagents for infectious disease marker(immunological method), IVD reagents for infectious disease marker(molecular diagnostics), etc. | 48 |
| IV | HIGH | IVD reagents for diagnosis of HIV/HBV/HCV/HTLV(immunological method), IVD reagents for ABO〮RhD blood typing(red cell agglutination), IVD reagents for diagnosis of HVI/HBV/HCV/HTLV(molecular diagnostics), etc. | 10 |
| TOTAL | 225 | ||
Premarket Approval Process
* Some Class II devices are subject to Approval
** ‘National Institute of Medical Device Safety Information (NIDS)
** ‘National Institute of Medical Device Safety Information (NIDS)
※NIDS is established pursuant to Article 42 of the Medical Device Act and has been entrusted with tasks related to the notification and certification of medical devices under Paragraph 2 of Article 44 of the Act by the Minister of Food and Drug Safety.
3.1 Certification of Class II Medical Devices
NIDS certifies ‘(Recognized) Substantial Equivalent(SE) devices’, and ‘Modified Devices’
NIDS certifies ‘(Recognized) Substantial Equivalent(SE) devices’, and ‘Modified Devices’
Class II
“SE Device” is a medical device(or an IVD) that is equivalent in ‘Intended Use’, ‘Mechanism of Action(MoA)’, ‘Raw Materials, ‘Performance’, ’Test Specification’(not applicable to IVD), ‘Instructions for Use’(not applicable to IVD) with previously approved/certified/notified medical devices. ※ For medical devices in Class II that were approved and certified for more than three times with the equivalent product, MFDS may publicly announce those as a ‘Recognized Substantial Equivalent(SE) devices’,
“Modified Device” is a medical device(or an IVD) that is equivalent in ‘Intended Use’, ‘Mechanism of Action(MoA)’, ‘Raw Materials(Limited to implanted/contacted devices & most of devices are not intended to operate electrically, not applicable to IVD)’ with previously approved/certified/notified medical devices, but not equivalent in ‘Raw Materials’(only applicable to IVD) ‘Performance’, ’Test Specification’ (not applicable to IVD), ‘Instructions for Use’ (not applicable to IVD).
“Modified Device” is a medical device(or an IVD) that is equivalent in ‘Intended Use’, ‘Mechanism of Action(MoA)’, ‘Raw Materials(Limited to implanted/contacted devices & most of devices are not intended to operate electrically, not applicable to IVD)’ with previously approved/certified/notified medical devices, but not equivalent in ‘Raw Materials’(only applicable to IVD) ‘Performance’, ’Test Specification’ (not applicable to IVD), ‘Instructions for Use’ (not applicable to IVD).
3.2 Approval of Class IIㆍIIIㆍIV Medical Deivces
NIFDS (affiliated agency of MFDS) approves 'New devices(Class Ⅱ)' and 'Class Ⅲ & Ⅳ Devices'
NIFDS (affiliated agency of MFDS) approves 'New devices(Class Ⅱ)' and 'Class Ⅲ & Ⅳ Devices'
Class II & III & IV
“New(novel) Devices” a medical device(or an IVD) that is not equivalent in ‘Intended Use’, ‘Mechanism of Action(MoA)’, ‘Raw Materials’(not applicable to IVD) with previously approved/certified/notified medical devices.
4. External Review
- MFDS has more than 300 commissioned external experts from various fields such as clinic, academia, industry, etc. participating in consultancy and & review for cutting-edge and new developed medical devices.
Overview of Device Business License
MFDS requires those who intend to produce a medical device in Korea, or who intend to import a medical device from overseas to obtain a manufacturing business license and an
import business license respectively.
Business license approval is handled by a concerning regional office of MFDS in the area where the business site is located.
As an applicant has to possess at least one product license in order to obtain a business license, approval processes of a business license and a product license are conducted contemporaneously.
Business license approval is handled by a concerning regional office of MFDS in the area where the business site is located.
As an applicant has to possess at least one product license in order to obtain a business license, approval processes of a business license and a product license are conducted contemporaneously.
Document Requirements
- A medical certificate by a doctor that proves an applicant is not a mental patient, or a medical certificate by a medical specialist that proves an applicant is adequate to be a manufacturer
- A medical certificate by a doctor that proves an applicant is not addicted to drugs or other toxic substances
- Documents that confirm qualifications of a quality manager
- A review for business license approval takes 25 days, and when a product license is finally approved (certified or notified), a regional office of MFDS issues the business license
Medical devices subject to tracking and control
52 medical device items are designated as the medical devices subject to tracking and control, which need to be traceable, as they can cause fatal
harm to a human body when an adverse event, or a defection occurs while using them. - 48 items that are implanted into a human body for over one year - Four (4)
life-sustaining items that can be used in places other than medical facilities
Preparation, Preservation, and Submission of Records (Article 50 of the Enforcement Regulations of the Medical Device Act)
- Medical device handlers shall prepare records on sales of medical devices by model and manufacturing unit, and users shall prepare records to make it possible to track patients who use a medical device.
| Matters to be stated (Article 50 of the Enforcement Regulations of the Medical Device Act) | ||
|---|---|---|
| Handlers | Manufacturers or Importers |
a. Quantity and date of manufacture/import by product name (or product group title if there is no product name)/model name/batch (applicable to manufacturers and importers only) |
| b. Quantity and date of sale/lease, name and address of seller/lessor or founder of a medical institution by product name (or product group title if there is no product name)/model name/batch (not applicable to repairers) | ||
| c. Other matters required to prevent harm to national healthcare and hygiene | ||
| Distributors or Lessors |
a. Quantity and date of sale/lease, name and address of seller/lessor or founder of a medical institution by product name (or product group title if there is no product name)/model name/batch (not applicable to repairers) | |
| b. Other matters required to prevent harm to national healthcare and hygiene | ||
| Repairers | a. Date of repair, and name and address of client by product name (or product group title if there is no product name)/model name/batch (applicable to repairers only) | |
| b. Other matters required to prevent harm to national healthcare and hygiene | ||
| Users(physicians or doctors, etc.) | a. Name, address, date of birth, and gender of a patient who uses a medical device subject to tracking and control | |
| b. Product name, and manufacturing number of a medical device subject to tracking and control, or information recognized as equivalent | ||
| c. Date of use of a medical device subject to tracking and control | ||
| d. Name and location of a medical facility using a medical device subject to tracking and control | ||
| e. Other matters required to prevent harm to national healthcare and hygiene | ||
The designation status of medical devices subject to tracking and control
- 48 items that are implanted into a human body for over one year (item no.1-48)
- Four (4) life-sustaining items that can be used in places other than medical facilities (item no.49-52)
| No. | Device name | No. | Device name |
|---|---|---|---|
| 1 | Pacemaker, cardiac, implantable[4] | 27 | Prosthesis, vascular, collagen-based[4] |
| 2 | Pacemaker electrode cardiac, implantable[4] | 28 | Prosthesis, vascular, heparin[4] |
| 3 | Prosthesis, valve, cardiac, composite[4] | 29 | Annuloplasty ring[4] |
| 4 | Prosthesis, valve, cardiac, biological[4] | 30 | Infusion pump, insulin, implantable[4] |
| 5 | Prosthesis, valve, cardiac, non-biological[4] | 31 | U-healthcare, insulin infusion pump, implantable[4] |
| 6 | Defibrillator, implantable[4] | 32 | Peripheral nerve electrical stimulation system, analgesic[4] |
| 7 | Infusion pump, electrically-powered, implantable[4] | 33 | Gait-enhancement electrical stimulation system, implantable[4] |
| 8 | Breast prosthesis, internal, gel-filled[4] | 34 | Incontinence-control electrical stimulation system, implantable[4] |
| 9 | Electrode/lead, defibrillator, implantable[4] | 35 | Stimulator, electrical, neuromuscular, scoliosis, implantable[4] |
| 10 | Prosthesis, temporomandibular[3] | 36 | Vagus nerve electrical stimulation system, coma arousal[4] |
| 11 | Prosthesis, temporomandibular, biological/biodegradable[4] | 37 | Carotid sinus nerve electrical stimulation system[4] |
| 12 | Prosthesis, mandibular[4] | 38 | Programmer, implantable stimulator, incontinence[4] |
| 13 | Prosthesis, mandibular, biological/biodegradable[4] | 39 | Bladder/bowel-evacuation electrical stimulation system[4] |
| 14 | Stent, vascular[4] | 40 | Adaptor, pacemaker lead, implantable[4] |
| 15 | Coronary artery stent[4] | 41 | Pacemaker repair kit[4] |
| 16 | Stent, iliac[4] | 42 | Hip prosthesis, internal, total , biodegradable[4] |
| 17 | Brain electrical stimulation system, psychiatric therapy[3] (implantable) | 43 | Knee prosthesis, internal, total biodegradable[4] |
| 18 | Stimulator, electrical, antiseizure[4] (implantable) | 44 | Prosthesis, shoulder, internal, total biodegradable[4] |
| 19 | Brain electrical stimulation system, antitremor[4] (implantable) | 45 | Prosthesis, wrist, internal, total biodegradable[4] |
| 20 | Stimulator, electrical, analgesic, implantable[4] | 46 | Prosthesis, elbow, internal biodegradable[4] |
| 21 | Electorde/lead, stimulator, analgesic, implantable[4] | 47 | Prosthesis, ankle, Internal total biodegradable[4] |
| 22 | Electrode for electrical stimulation system, implantable[4] | 48 | Hip prosthesis, internal, total (approximalsurface metal)[3] |
| 23 | Circulatory assist system, artificial heart[4] | 49 | Ventilator, continuous, home-use[3] |
| 24 | Diaphragm/phrenic nerve electrical stimulation system[4] | 50 | Defibrillator, lowpowered[3] |
| 25 | Prosthesis, vascular, central[4] | 51 | Defibrillator, highpowered[3] |
| 26 | Prosthesis, vascular, peripheral[3] | 52 | Patient monitoring system, respiratory, radiation procedure(always use)[2] |